The Global Periodic Table and a Proposed Standard Classroom Version
brian.gregory6@gmail.com
1. Mathematical Aufbau 1
Fig. 1 represents the first ten electron shells sorted first by size (principal quantum number n) and then by shape (angular quantum number l ).
Column ml shows magnetic quantum numbers -l to l with negative numbers in red.
Each of the 2l +1 terms of the ml string represents a unique orientation of the (n, l ) orbital—with 2 electrons per orbital
the total subshell capacity is 2(2l +1) = 4l +2.
A simple algorithm loads non-repeating positive integers into subshell strings of length 4l +2.
If we want the algorithm to model the subshells of the periodic table, after a promising start it fails at atomic number 19.
In the periodic table potassium opens the 4s subshell, but sorting by n puts integer 19 in the 3d subshell string.
In a one-electron system like the hydrogen atom energy depends only on n, so sorting by n is appropriate and the 3d subshell is preferentially occupied.
But in multi-electron systems orbital energy increases with increasing n and with increasing l .
Column 4l +2 is the DNA for building the subshells of the periodic table and beyond, but the DNA is out of sequence.
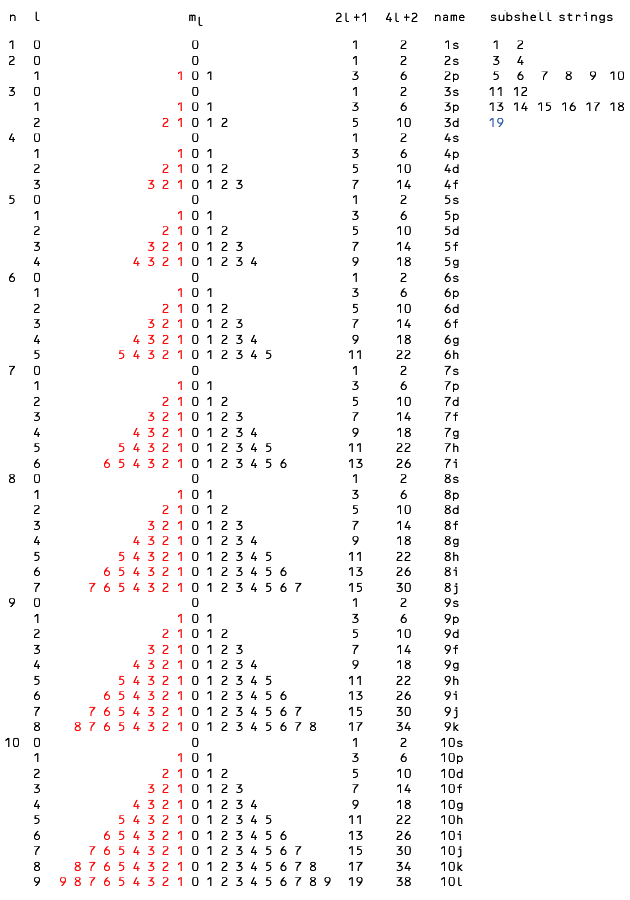
Fig. 1
2. Mathematical Aufbau 2
Angular quantum number l corresponds to the number of orbital angular nodes, the planar or conical surfaces through the nucleus with zero probability of finding the electron.
As the number of angular nodes increases, electrons are pushed farther away from the nucleus.
[Quoting Richard Feynman, "We would like to mention one particular feature of the wave functions for higher l :
for l > 0 the amplitudes are zero at the center. That is not surprising, since itís hard for an electron to have angular momentum when its radius arm is very small.
For this reason, the higher the l , the more the amplitudes are 'pushed away' from the center." (Feynman Lectures on Physics, Volume III, Section 19-5)]
To recognize the energy contributions of both n and l we sort the subshells according to Madelung's rules—first we rank them by n+l so that 3d (3+2) outranks 4s (4+0) as desired,
and within ranks we sort by n as before so that 4s (4+0) outranks 3p (3+1).
The algorithm now blows through number 19 and all the known elements to generate all possible subshell strings (Fig. 2).
The integers may be interpreted as atomic numbers but not as electrons.
The sort and algorithm do not predict that the differentiating electron in feynmanium—electron 137—enters a g orbital, for example,
but it states unequivocally that atomic number 137 is the next to final term in the subshell string 5g* = [121, 138].

Fig. 2
The triangular arrays in column ml are now inverted due to the complementary nature of n and l
within an n+l rank—as n goes up l must come down.
The length of a period—the combined lengths of its subshell strings—grows by the familiar pattern: 2-8-8-18-18-32-32-50-50-etc.
Odd-numbered ranks start a new countdown from l to 0.
Even-numbered ranks repeat the previous countdown, only this time with a higher n, so period lengths after the first come in duplicate.
3. The n+l Sort vs. The n+l Rule
The n+l rule is introduced in the context of aufbau to explain the order in which subshells are filled, which it does with better than 80% reliability.
The n+l sort is framed in the context of spreadsheets to prioritize the combined contributions of two columns of data,
like a bank ranking its customers by account balance plus heavy usage. As a basic spreadsheet function the n+l sort is 100% reliable.
The 4l +2 algorithm then partitions the positive integers into disjoint intervals of length 4l +2, also with 100% reliability.
The sort and algorithm present a very weak version of the n+l rule—they categorically establish the subshell strings but say nothing about the order in which actual subshells are filled.
4. Subshell Strings vs. Subshells
The 4l +2 string holds just enough slots for a subshell without addressing the order in which it is filled.
Consider the string 4d* = [39, 48], shown in the bottom row of the table below.
As we move through the string from yttrium (39) to cadmium (48) the 4d subshell grows erratically, as shown in the top row.
The system of valence electrons always includes the highest s subshell so we insert a row for 5s, which in this case is close enough in energy to the 4d that it is selectively occupied for overall stability.
The third row adds the complementary rows term-by-term for the total valence electron count.
Here the totals naturally coincide with the chemical groups 3 through 12 in the periodic table.
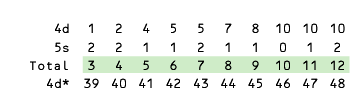
In string 5f* = [89, 102] shown below, actinium (89) and thorium (90) have no 5f electrons—the differentiating electrons enter the complementary 6d subshell.
Here the totals do not coincide with the chemical groups 3 - 12, which are exclusive to the d-block.
Actinium (89) and thorium (90) have configurations indicative of groups 3 and 4, respectively, but they are on a different trajectory as subsequent electrons increasingly occupy the 5f subshell.
Totals in string 3p* = [13, 18] indicate groups 3 - 8, while the modern groups are 13 - 18.
And just for fun here's 1s* = [1, 2]:

The valence electron totals suggest a primary or ancestral classification scheme that is later modified to form the modern chemical groups of the periodic table.
5. The Global Periodic Table
Fig. 5-1 below shows the subshell strings aligned in columns based on the total pool of valence electrons as described above. This is the global periodic table. Each column constitutes a global group. Each term in column 1 launches a global period. The global periodic table is a purely mathematical matrix that assigns precise row and column coordinates to all positive integers but makes no predictions as to the order in which subshells are filled.
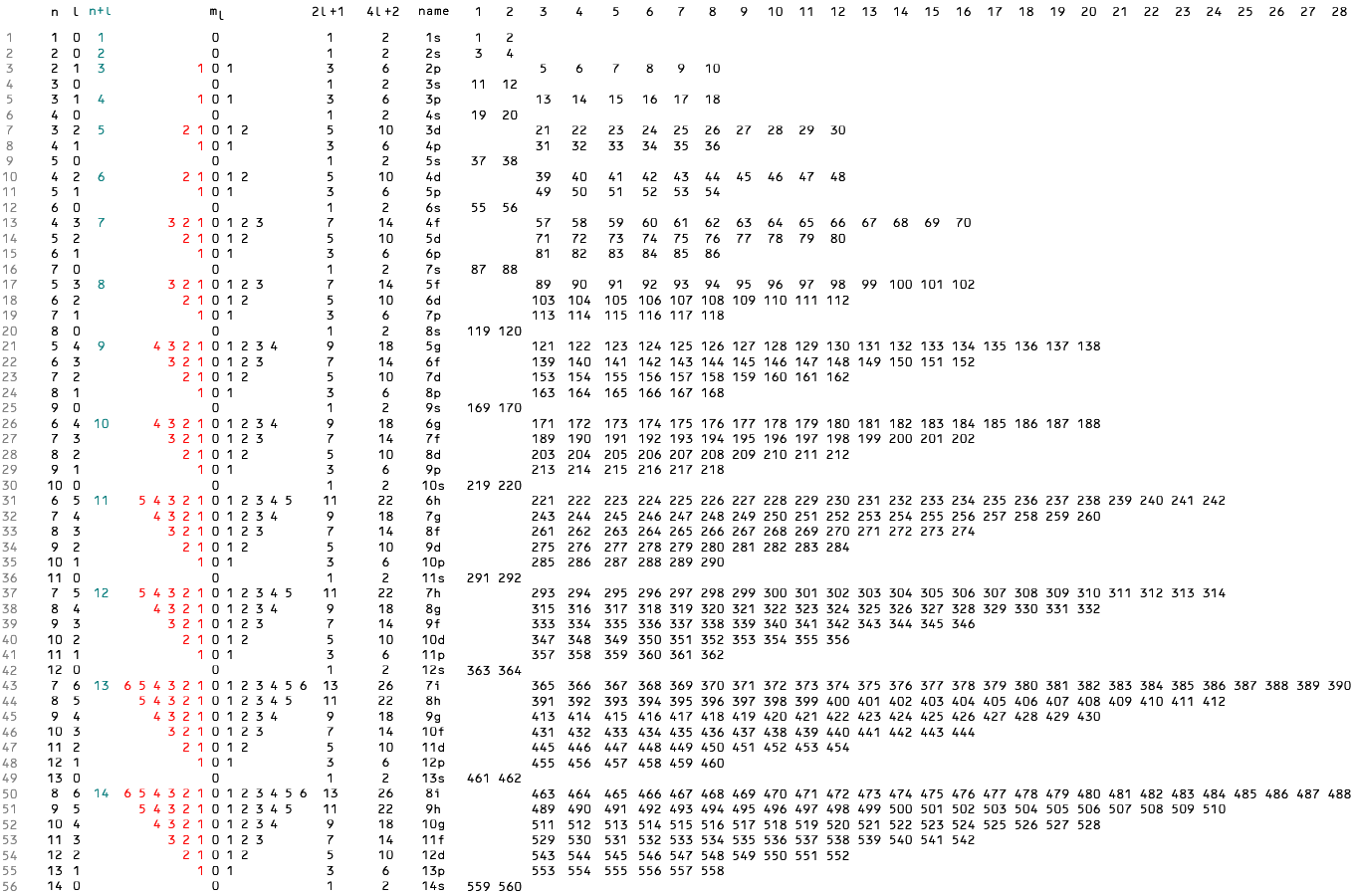
Fig. 5-1
The "local" periodic table of known elements is the truncated global table ending with integer 118 (Fig. 5-2). Up to roughly global group 7, global group number naturally coincides with the highest oxidation state, otherwise membership in a global group doesn't necessarily imply any similarity in chemistry. Neon (10), iron (26) and plutonium (94) fall in global group 8 because they have exactly 8 electrons in the emerging sp, sd, or sf subshell system: Ne = [He]2s22p6, Fe = [Ar]4s23d6, Pu = [Rn]7s25f6. Oxygen (8), chromium (24) and uranium (92) fall in global group 6 with the configurations O = [He]2s22p4, Cr = [Ar]4s13d5, and U = [Rn]7s25f36d1.
Fig. 5-2
The Global Periodic Table through Z=118
(Standard Form)
Subshells of the same length constitute a block. Sort by l and then n to switch to block form (Fig. 5-3).
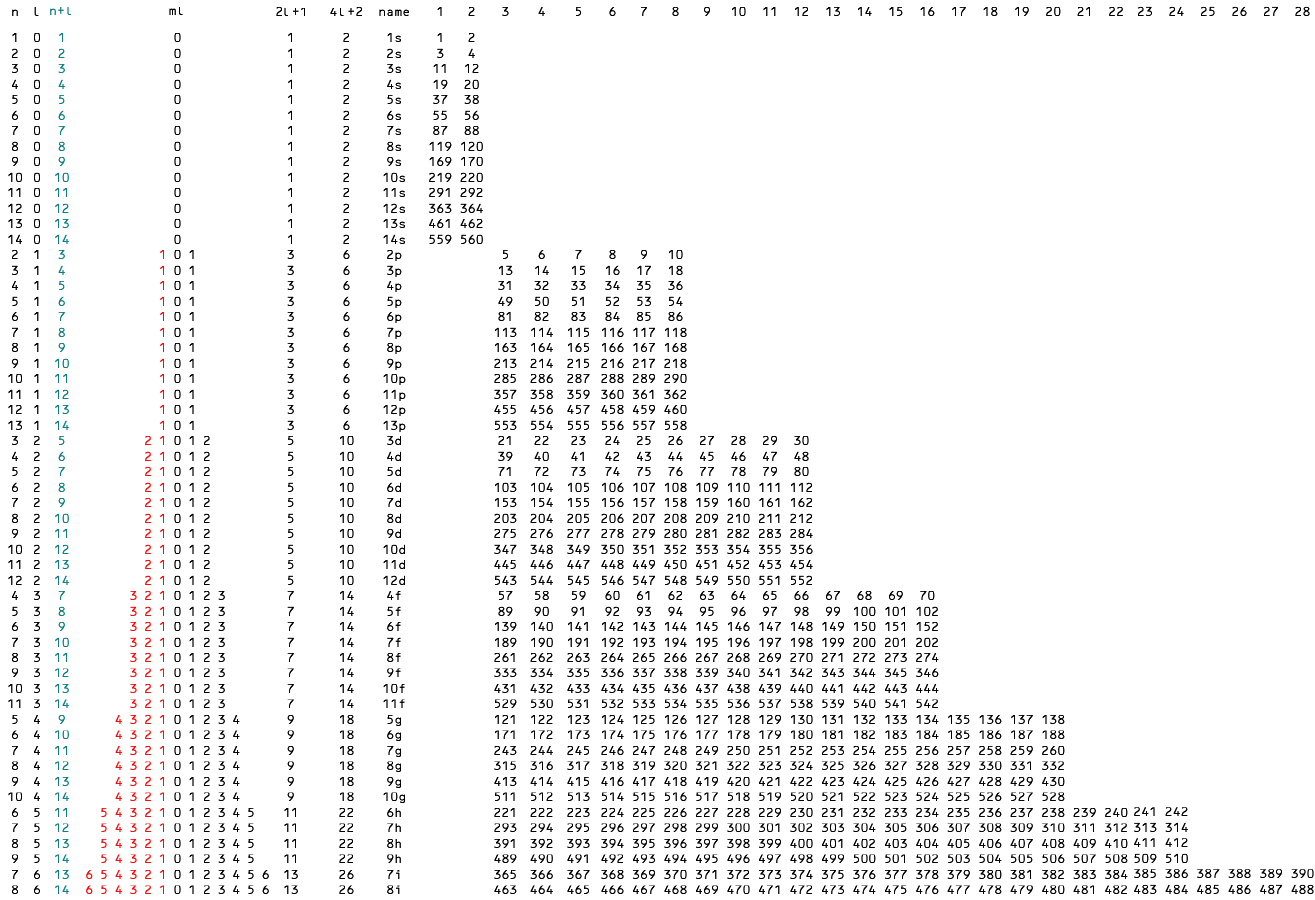
Fig. 5-3
Fig. 5-4
The Global Periodic Table through Z=118
(Block Form)
6. Block Orderings
Fig. 6-1 zooms to Z = 218 with color-coded blocks and the known elements in bold. At this stage no blocks share a vertical border.
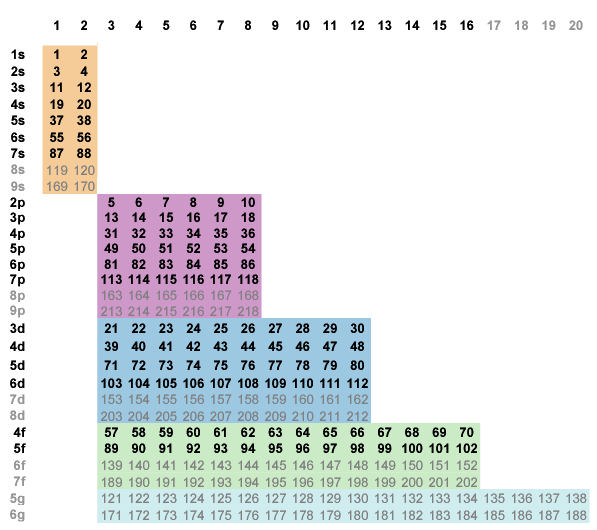
Fig. 6-1
A block ordering is a vertical border with one or more pairs of consecutive integers. For example, slide the s-block down until the pairings (4,5) and (12,13) lock in place, creating a partial ordering along the new border s|p (Fig. 6-2).
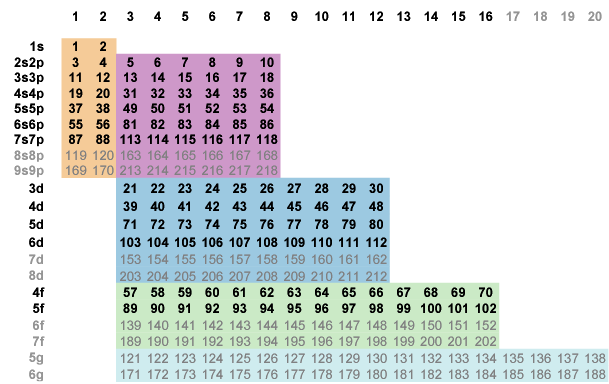
Fig. 6-2
Now slide the s-block up and right to establish a fully ordered border p|s (Fig. 6-3). Fully ordered borders come at a price, as global groups 1 and 2 now fall in columns 9 and 10.
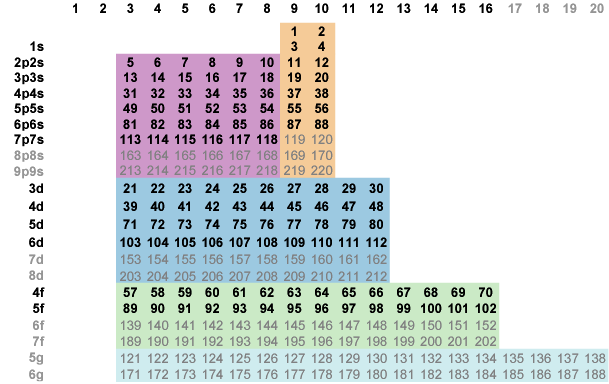
Fig. 6-3
Insisting on fully-ordered borders gives the left-step periodic table (Fig. 6-4). To complete the full ordering here we'd have to bring up the g-block for a 50-column left-step table. Long form tables erase all global periodicities—the repeating patterns in valence electron counts across groups responsible for the original global structure. Here the global groups are indicated only in the awkward column numbering.
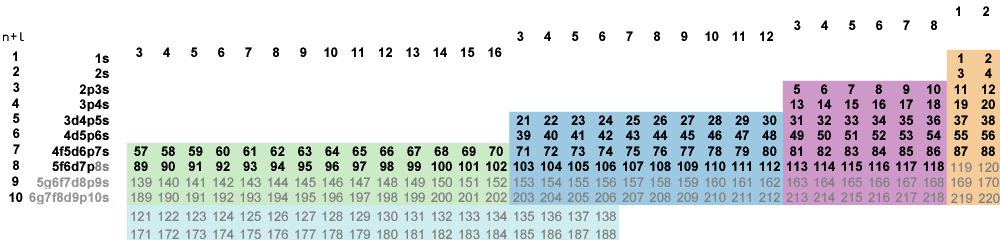
Fig. 6-4
7. Global Groups to Chemical Groups
Global groups are strictly determined by the quantum numbers under the n+l sort, the 4l +2 algorithm, and the two-column advance for nonzero l (Fig. 5-1).
Chemical groups arise from a simple block ordering.
Starting with Fig. 6-2 above, swipe the p-block ten columns right and drop the s and p-blocks for a partially ordered
s|d and a fully ordered d|p (Fig. 7-1).
In the upper table (s|d|p) the global groups have magically morphed into the modern chemical groups
(except for He in group 2).
This is a substantial ordering—the first five rows now coincide with the first five periods with each term in sequential order.
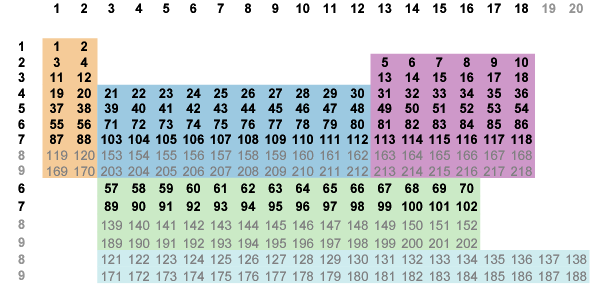
Fig. 7-1
Let's drop the theoretical elements and wall off the f-block from the chemical groups of the upper table:
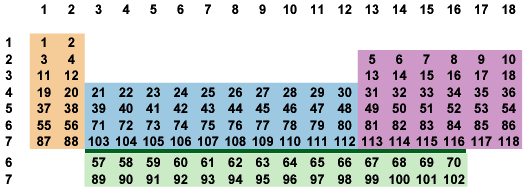
Fig. 7-2
All periods start in the first column and all but the first end in the last column. If we insist that all periods start in the first column and end in the last column we get the arrangement in Fig. 7-3. The "period rule" fixes hydrogen in group 1 and helium in group 18 without invoking electron configuration or chemical behavior.
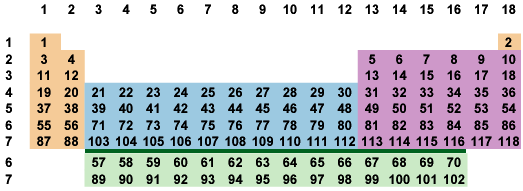
Fig. 7-3
8. A Proposed Standard Classroom Periodic Table
Today there are three competing versions of the classroom periodic table and each tells a slightly different story.
The discrepancies occur in the bottom half of group 3 and at the start and end of the f-block, which is cut from the upper table and pasted below it.
- Version 1
group 3 = Sc/Y/Lu/Lr
f-block = La/Ac - Yb/No
Example: WebElements
- Version 2
group 3 = Sc/Y/La/Ac
f-block = Ce/Th - Lu/Lr
Example: Royal Society of Chemistry
But click "group 3" in the example and it shows only Sc/Y. La/Ac are shown in group 3 in the d-block but color-coded to indicate membership in the f-block.
- Version 3
group 3 = Sc/Y/f-block
The entire f-block is assigned to group 3 in the d-block.
f-block = La/Ac - Lu/Lr
Example: IUPAC
The confusion results from the false premise that the 18-column periodic table is derived from a "superior" 32-column periodic table by cutting and pasting the f-block below it.
Starting with the block configurations in Fig. 5-4, we derived the 18 chemical groups via block orderings leaving the f-block untouched.
We need two additional block orderings (s|f and f|d) to fit the f-block into the upper matrix.
In this analysis we'd have to cut and paste the f-block from an 18-column table to make a 32-column table, not the other way around.
group 3 = Sc/Y/Lu/Lr
f-block = La/Ac - Yb/No
Example: WebElements
group 3 = Sc/Y/La/Ac
f-block = Ce/Th - Lu/Lr
Example: Royal Society of Chemistry
But click "group 3" in the example and it shows only Sc/Y. La/Ac are shown in group 3 in the d-block but color-coded to indicate membership in the f-block.
group 3 = Sc/Y/f-block
The entire f-block is assigned to group 3 in the d-block.
f-block = La/Ac - Lu/Lr
Example: IUPAC
Everyone is free to use any version of the periodic table they desire depending on their specialty or personal preference, but if the goal is to adopt a definitive standard periodic table for classrooms and textbooks based on maximal scientific, pedagogic and heuristic value, I propose the arrangement in Fig. 7-3 above (or click the printable version below).
The transition will be seamless as Fig. 7-3 is practically identical to the three periodic tables currently in use—it just sharpens the edges around group 3 and the f-block. Group 3 consists precisely of Sc/Y/Lu/Lr with La/Ac walled off below in the related global group 3 (based on electron count). The f-block starts with La/Ac in global 3 and ends with Yb/No in global 16. The lanthanoids and actinoids don't float randomly below the upper table—they are fully integrated into a single matrix based on the same physics and math as the other blocks. There are no ambiguities in this arrangement—every element falls in a fixed row and column coordinate within a single matrix. It is fully derived from purely mathematical operations independent of the controversial and often contradictory arguments based on physical and chemical properties and electron configuration. If members of a group happen to share certain physical and chemical properties, that is just a bonus! The 18-column format best reflects the major periodic trends in electronegativity, ionization energy, electron affinity, atomic radius, melting point, and metallic character, consistent with the current textbooks and classroom materials. As more elements are discovered we simply lower the f-block to accomodate them, but the f-block must span columns (global groups) 3 - 16.
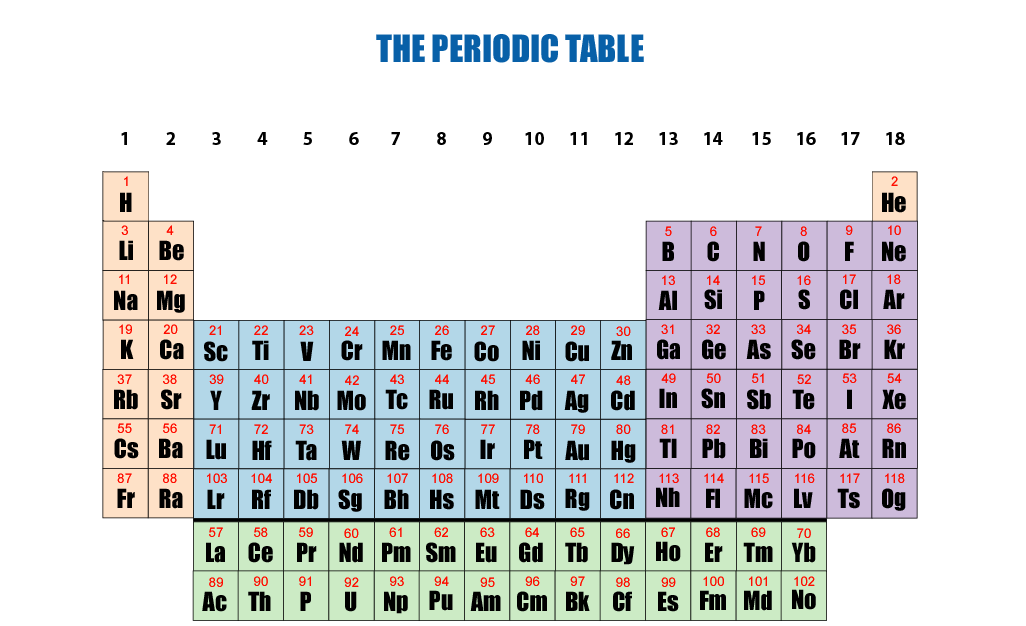
Proposed Final Form of the Standard Periodic Table